co2 electron geometry and molecular geometry|Molecular Geometry Of CO2: How Lewis Structure : Cebu One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement . Tingnan ang higit pa European Roulette is an exciting game for those who want to try their luck. Beautiful graphics and user-friendly interface are waiting for you. Place your bets, ladies and gentlemen!
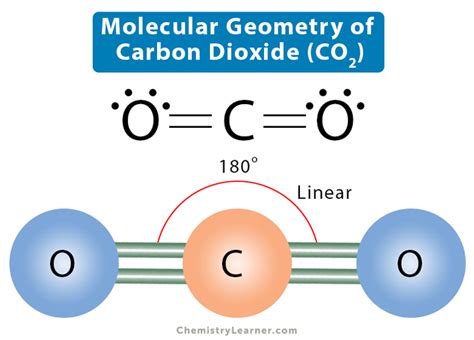
co2 electron geometry and molecular geometry,CO2 Molecular Geometry. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. As a result, there are no lone pairs of electrons, . Tingnan ang higit paOne needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement . Tingnan ang higit paThe electronic configuration of the Carbon atom in its ground state is 1s22s22p2, and that of an Oxygen atom is 1s22s2p4. When the electrons are in an excited state, they jump to other orbitals. In its excited state, the atom’s electronic configuration becomes . Tingnan ang higit paThe molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central . Tingnan ang higit paFigure \(\PageIndex{1}\) shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When . In this video we look at the electron geometry for CO2 (Carbon Dioxide). Because the Carbon dioxide molecule has two electron domains (two oxygen atoms and . For example, in CO2, carbon needs 6 electrons to fulfill the octet, whereas oxygen needs only 2 electrons. Now, let us quickly go through the steps for creating a . Example \(\PageIndex{1}\): Predicting Electron-pair Geometry and Molecular Structure. Predict the electron-pair geometry and molecular structure for each of the following: carbon dioxide, CO .co2 electron geometry and molecular geometry Molecular Geometry Of CO2: How Lewis Structure CO2 Molecular Geometry & Shape. In a CO2 molecule, the carbon atom is in the center double bonded with two oxygen atoms by each side. Both oxygen atoms have two lone pairs of nonbonding .We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom, ignoring .Molecular Geometry of CO 2. CO 2 molecular geometry is based on a linear arrangement. The presence of a sigma bond and valence electron pairs repelling each other force them to move to the opposite side of the .
co2 electron geometry and molecular geometryElectron Geometry VS Molecular Geometry - The key difference between electron geometry and molecular geometry is that electron geometry is found by utilizing both single electron combines and bonds in a particle, .

After calculating the total number of valence electrons around the CO 2 molecule, it is easier to distribute them around the carbon and oxygen atoms and get an appropriate Lewis structure. The Lewis structure of . The 2pz orbitals of carbon and three O atoms are available for delocalized pi bonding. We have two electrons filling bonding molecular orbital, four filling non-bonding MOs. Therefore, the six pi .Determine the electron geometry (eg) and molecular geometry (mg) of Cl3+. eg = trigonal planar, mg = trigonal planar O O eg = tetrahedral, mg = trigonal planar O eg = tetrahedral, mg = trigonal bipyramidal O eg = . Hydrogen Cyanide has geometry like AX2 molecule, where A is the central atom and X is the number of atoms bonded with the central atom. As Carbon is bonded to two atoms, it follows the .Molecular Geometry Of CO2: How Lewis Structure Why is the molecular geometry of CH4 is same as its electron geometry? The molecular geometry of CH4 is tetrahedral and its electron geometry is also tetrahedral. Because as per VSEPR theory, molecular shape considers only bond pairs; While electron geometry considers bonded atoms as well as lone pairs present on the .
Carbon Monoxide is a diatomic molecule with a triple bond between C and O and one lone pair of electrons on each atom. And since it only has two atoms, it has a linear molecular geometry. Carbon and Oxygen forms one sigma bond and two pi bonds.This online quiz is intended to give you extra practice in identifying the molecular and electron geometry of chemical compounds using VSEPR theory. Select your preferences below and click 'Start' to give it a try! Number of problems: 1 5 10 25: Question types (select at least one): Molecular shape Electron geometry AXE notation This, in turn, makes these electrons readily available upon excitation. Carbon Tetrafluoride comprises four Fluorine atoms and a single Carbon atom. Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s 2 2p 2. Therefore, a single carbon atom contributes: 4 x 1 = 4 Valence Electrons.Determine the electron geometry (eg) and molecular geometry (mg) of CBr3+? There are 3 steps to solve this one. 100 % .
CO2 has a total of 16 valence electrons (carbon has 4 and oxygen 6 valence electrons). CO2 has a linear molecular geometry with a bond angle of 180° on a plan. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. Carbon dioxide has an sp hybridization type because the steric number of central carbon is 2.

When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles. . (CO2) and molecules with a single and triple bond (HCN). H-Be-H: O=C=O \(H-C\equiv N\) BeH 2: CO 2: HCN: Note, Beryllium can have less than an octet . So, carbon needs four electrons to complete its octet. Let us move to hydrogen, where its atomic number is one, and electronic configuration is 1s1. . Molecular Geometry of CH2O. The CH2O is a .
Molecular Geometry: The molecular geometry of the compound refers to the representation of the structure in three-dimensional form. It is based on several principles such as the valence shell electron pair repulsion, Lewis dot structure and octet rule.
Carbon dioxide is a chemical compound made when carbon combines with oxygen in a 1:2 ratio. It is a gas at room temperature and pressure and it is environmentally significant as a driver of climate change. The atomic number of carbon is 6, where its electronic configuration is 1s2 2s2 2p2. To achieve a stable state, the p shell needs to accommodate 6 valence electrons. So, the total number of valence electrons in carbon is 4. . Molecular Geometry of Carbonyl Fluoride (COF2) Molecular geometry is a 3D diagrammatic way of studying .
The molecular geometry of C2H4 is trigonal planar and its electron geometry is also trigonal planar according to VSEPR (Valence shell electron pair repulsion theory). As e ach carbon in the C2H4 molecule has Sp² hybridization and with two hydrogens it makes the structure look like a triangular planar which is two-dimensional. The central atom in the CH2O molecule is carbon which also needs 8 electrons in its outer shell for completing the octet. In the 4th step structure, we see the carbon atom has 6 electrons in its outer shell in form of three single bonds but it needs 2 more electrons for completing the octet. . Therefore, both electron and molecular .
Determine the electron geometry (eg), molecular geometry (mg), and polarity of HIO2. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
The carbon in methane is said to have a tetrahedral molecular geometry AND a tetrahedral electronic geometry. Say what? Molecular vs Electronic Geometry. When looking at the shape of a molecule, we can look at the shape adopted by the atoms or the shape adopted by the electrons. Every electron pair within methane is bound to .
co2 electron geometry and molecular geometry|Molecular Geometry Of CO2: How Lewis Structure
PH0 · Molecular Geometry Of CO2: How Lewis Structure
PH1 · Electron Geometry for CO2 (Carbon Dioxide)
PH2 · Electron Geometry VS Molecular Geometry
PH3 · CO2 Lewis Structure, Molecular Geometry, Molar Mass & Hybridization
PH4 · CO2 Lewis Structure, Molecular Geometry, Molar
PH5 · CO2 Lewis Structure, Molecular Geometry and Hybridization
PH6 · CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagr
PH7 · CO2 Lewis Structure, Hybridization, Molecular Geometry, and
PH8 · CO2 Lewis Structure,
PH9 · 8.6: Molecular Geometries
PH10 · 5.9: Molecular Geometry
PH11 · 10.2: VSEPR Theory